Oysters, Selective Pressures, and Antibiotic Resistance in the Mississippi Delta
In recent years, in addition to its position as a pillar of New Orleans cuisine, the humble oyster has also taken on another, more troubling role—serving as an indicator of water contamination in the Mississippi River Delta and the Louisiana Gulf. In this contribution, Flavio D’Abramo, a researcher at the Max Planck Institute investigating organisms’ biological changes in relation to different kinds of environmental stresses, identifies the links between oysters, industrial pollution, and antibiotic resistance: the biological hallmark of the Anthropocene.
Oysters have been known to humankind for many centuries. In 1877, the German professor Karl August Möbius proposed the term “biocenosis” or “living community” (Lebensgemeinde)1 while trying to understand the flourishing of oyster’s beds in the Kiel Bay, which was famous for this kind of crustacean delicacy during the eighteenth century. The term indicates the dependence of all the organisms of a community upon one another. Moreover, it links the organisms to factors of their environment, such as water temperature and salinity, and it accounts for the number of organisms of each species. Möbius witnessed this interdependence by counting more than three hundred organisms sheltered within the valves of just two oysters. This prompted him to warn the local community of fishermen and traders to consider this delicate interconnection so as not to follow the example of Cancale in France, where oyster populations were depleted by overfishing.2 As a result of this, in France the ecological niche previously occupied by oysters was taken up by mussels and cockles, a change that for years prevented any possible reestablishment of the oyster population.
As Möbius’ studies show, almost two centuries ago, the wild oysters that called French shores home were put in danger by humans. By contrast, in New Orleans, restaurants protect customers with a weakened immune system by showing them warnings associated with the consumption of oysters from the area, which can cause severe illness in some people and even death. The reason for such caution lies in the Louisiana industrial development since the beginning of the twentieth century when oil refineries were first built and started to exert environmental, selective pressures on microorganisms’ metabolism to eventually contribute to the rise of antibiotic resistance.
The key dynamics explaining the different role played by French oysters during the nineteenth century compared with oysters currently being fished in the Gulf of Mexico relates to selective pressures imposed by the industry at the microscopic scale. Specifically, this affects bacteria, which have delicate and highly dynamic equilibria, and are thus extremely sensitive to external conditions. Most importantly, since bacteria are necessary for all living beings to develop and survive, and since they can rapidly communicate to each other, their status directly impacts human health. Lateral gene transfer (aka horizontal gene transfer), which is the molecular, evolutionary mechanism allowing a rapid “communication” across bacteria and between bacteria and viruses, and which explains antibiotic resistance, has been studied for almost seventy years and is the biological hallmark of the Anthropocene.3 Bacteria are the material, elemental interface between multicellular organisms and the environment. They can quickly change their genome in cases of external stress to develop resistance to dangerous, environmental factors. Synthetic chemicals such as antibiotics used in aquaculture and livestock, or phosphorus and nitrogen contained in fertilizers, play a major role in the genetic variation of bacteria—a dynamic that can easily lead bacteria to develop and share resistance to these elements.
This image captured on July 20, 2019 by the European Space Agency’s Corpenicus Sentinel-2 satellite of the shows the chlorophyll produced by Cyanobacteria, tinting the water of the Baltic Sea. Cyanobacteria, which with other organisms compose phytoplankton, use chlorophyll for photosynthesis though which atmospheric carbon dioxide is converted in oxygen. Cyanobacteria bloom on agricultural and industrial run-off, such as phosphorus, and on water with a high temperature. When they reach a certain mass, bacteria create dead zones where fish cannot survive and elicit the proliferation of algae that is dangerous for humans and other animals. Contains modified Copernicus Sentinel data (2019), processed by ESA, CC BY-SA 3.0 IGO
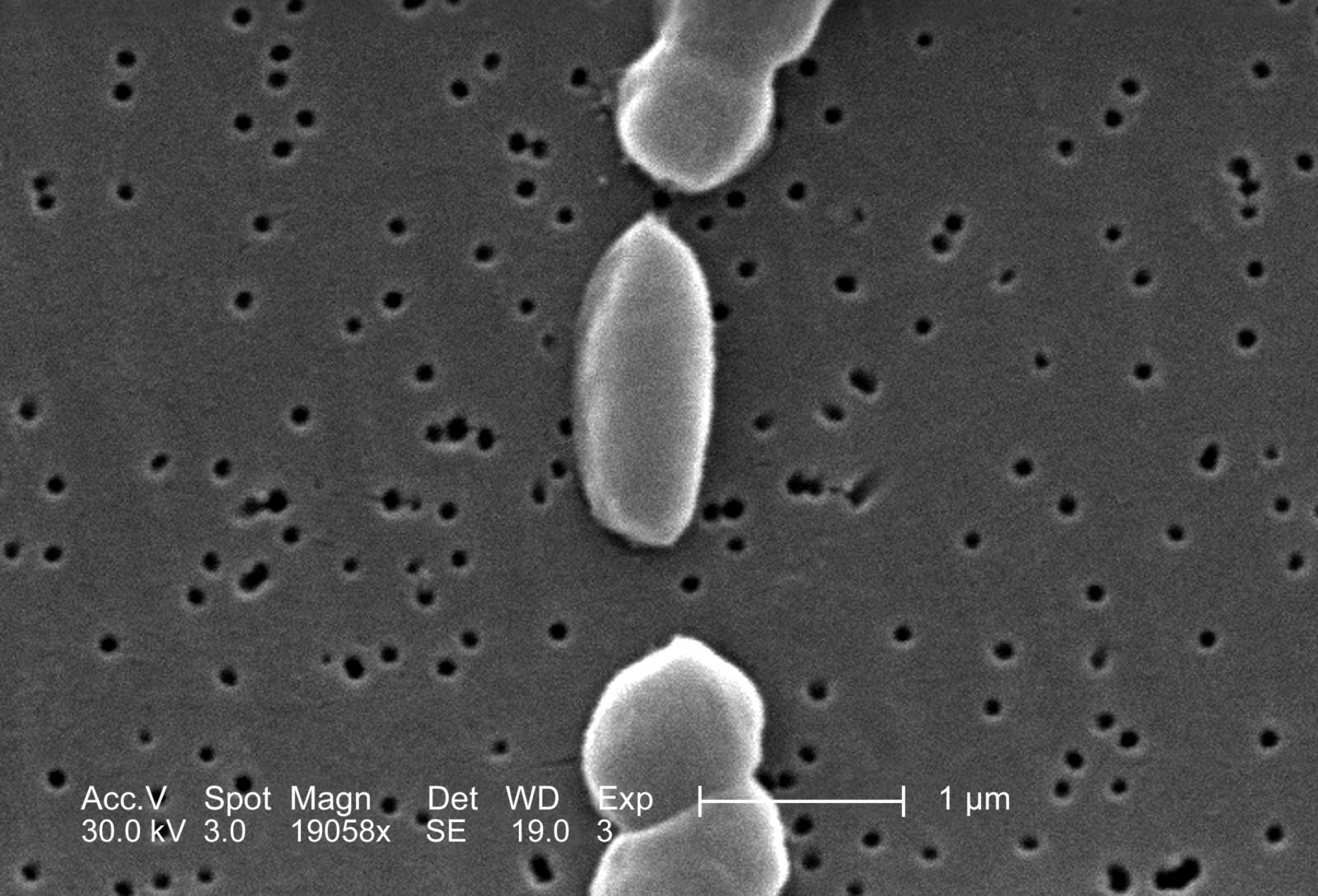
The presence of antibiotic-resistant bacteria in oysters such as those found in New Orleans restaurants derives from the constant contamination of water of Louisiana Gulf and the Mississippi River Delta. This happens via agricultural runoff and wastewater treatment plants present throughout the long journey of the river. These human activities spill antimicrobials, heavy metals, and elements such as nitrogen and phosphorus into the water, all acting as co-selecting agents to elicit the development and spread of antimicrobial-resistant aquatic bacteria.4 In 2007, a team of researchers from Louisiana State University found antibiotic resistance in Vibrio parahaemolyticus and Vibrio vulnificus bacteria extracted from oysters sampled in the Mississippi River, the Louisiana Gulf, and in retail raw oysters.5 A decade later, in 2018, bacteria resistant to carbapenem, monobactam, penicillin, tetracycline, sulphonamide, and cephalosporin antibiotics, and related resistance genes were consistently found in the coastal water of Louisiana.6 Both wild and farmed, the fish and shellfish living in these waters metabolize the aquatic bacteria and therefore acquire antibiotic resistance.
Antibiotic resistance and the risk of ill health it provides in humans is a phenomenon that has increased at the planetary scale during the last century, and has been known about since the introduction of Penicillin a century ago.7 Antibiotics such as sulphonamides, which are by-products of coal-tar, are part of the economy of heavy industry, and are used in several sectors, from the agroindustry to public health. The multiple selective pressures elicited by synthetic molecules of various kinds creates so-called superbugs such as Acinetobacer baumanii, multidrug-resistant Pseudomonas aeruginosa, methicillin-resistant Staphylococcus aureus (MRSA) and carbapenem-resistant Enterobacteriaceae. These bacteria are resistant to most, if not all, currently known antibiotics.
In countries like the USA, this health emergency is responsible for tens of thousands of deaths every year and for hundreds of thousands of sick individuals. Antibiotic resistance takes place in hospitals, where patients, doctors and nurses are in the midst of a planetary, environmental issue and is it this group to whom blame is often erroneously attributed. The real causes are in fact an entanglement of environmental factors that derive from the commodification of territories occupied by petrochemical refineries, livestock farming, and intensive agriculture, all of which is enhanced by supply chain systems. In the New Orleans area, both the environmental degradation and major social issues are a product of the scale of industrial programs that directly impact individuals and communities. As described by John W. Day and colleagues, since the 1930s the Mississippi River Delta:
has been profoundly altered by humans with respect to hydrology, sediment supply, sea-level rise, ecology, and land use that directly affect sustainability, especially in the context of global change forcings […] and is in the process of a physical, ecological, and societal collapse.8
One of the many industrial plants found along Louisiana’s “Cancer Alley,” captured during the Risk/Equity seminar field trip, as part of the Anthropocene River Campus, November 2019. Photograph by Aurora Levins Morales
In the Mississippi River Delta, antibiotic resistance is entangled with the ecological disruption elicited by the intensive activities of the petrochemical industry.
The CDC, the U.S. Center for Disease Control Prevention, contains antibiotic resistance in Louisiana and other states through constant monitoring of its trends by asking hospitals to run a yearly antibiogram, which is a summary of the most important antibiotic resistance patterns for individual hospitals for the past year.9 The scientific literature about the environmental causes of antibiotic resistance in Louisiana is, not surprisingly, in short supply. The first (above-mentioned) study to analyze the presence of antibiotic-resistant bacteria and antibiotic resistance genes in waters of southeast Louisiana dates back to 2018.10
One reason for this lack of scientific studies on antibiotic resistance is the challenge posed by putting together phenomena spanning across different time scales. In ten hours, a small colony of one million bacteria can produce around three hundred genetic mutations. At a different temporal scale, the selective pressure of the industry that elicits bacterial mutations has been taking place for the past hundred years or so, beginning when the first products of oil refineries were introduced in the environment. This intertwining of different bacterial and industrial rhythms causes an exponential leap, one in which millions of bacterial mutations foment the planetary emergency of antibiotic resistance, which slowly kills many hundreds of thousands of people every year—without even counting those living in the Global South who are not represented by censuses undertaken by international organizations.
An additional difficulty arises from the fact that the causes of antibiotic resistance are typically rooted in activities whose dynamics follow non-linear pathways with downstream flows that are difficult to unravel. Moreover, grassroots movements, which often push public institutions and regulatory agencies to consider and address issues of public health in Louisiana are focused on other major health threats. For example, grassroots movements such as Cancer Alley, the Louisiana Environmental Action Network (LEAN), and the Concerned Citizens of St. John concentrate their forces upon negotiating with public institutions, experts, and petrochemical corporations about the environmental, geographically circumscribed conditions causing many individuals in the region to develop cancer, asthma and other health issues.
Antibiotic resistance in the New Orleans area and southeast Louisiana is in fact a geographically dispersed process that concerns some 3,700 kilometers of river and multiple sites of origins. Even if antibiotic resistance is mainly recorded in hospitals, its roots lie in several locations, some of them thousands of kilometers away from the problem’s acme. Therefore, the geographically situated dimension of pollutants that cause the ill health of local communities and which shape the identity and practices of grassroots movements, gets lost in the geographically scattered dimension of antibiotic resistance. The long journey of the Mississippi River is a major challenge when attempting to track and control causes of antibiotic resistance, as in its course it gathers the chemical outputs of ten states, which then end up in the dead zone of the Mississippi River Delta—where the superbugs flourish.
In the short and medium-term, the endeavors of biomedical research and its translation (from lab bench to bedside) represent an effective, biomedical form of help for patients infected by antibiotic-resistant bacteria—through the discovery of new antibiotics, for instance. However, bacterial dynamics are so fast and ineffable that both precise prediction and interventions against bacterial resistance are doomed to failure. To put it another way: in the long run, industrial pollution needs to be considered as a factor that will always elicit the resistance of bacteria. A worthy social, scientific and political challenge to seriously consider the historical and biological trajectories of antibiotic resistance therefore requires an appreciation of bacterial dynamics as key, elemental processes necessary for the flourishing of all individuals and communities of animals and plants and that, at the same time, constrain human societies to both nature and industrial development. This is a challenge that calls for a change of political and scientific regimes of blind industrialization, demanding that they instead consider nature as partner.